TRACEABILITY THROUGH AUTOMATIC COUNTING IN THE PHARMACEUTICAL SECTOR
The balancing unit is used to balance products during production and to record samples when carrying out a media fill. All counting processes are documented (digitally and in paper form). Individual processes enable the printing of container cards, the comparison of counting results and interlocks in the event of an error. Intuitive operator guidance guarantees an optimum workflow. Counting results are visualized clearly and comprehensibly. With a processing speed of approx. 180 trays per hour, an ideal process time is achieved.
Manual processes are often very error-prone and are no longer permitted in many areas. Operators are
Estimates (used to count the total number of vials and cylinder ampoules on a pallet) are used to add up the maximum number of objects and the number of trays per pallet.



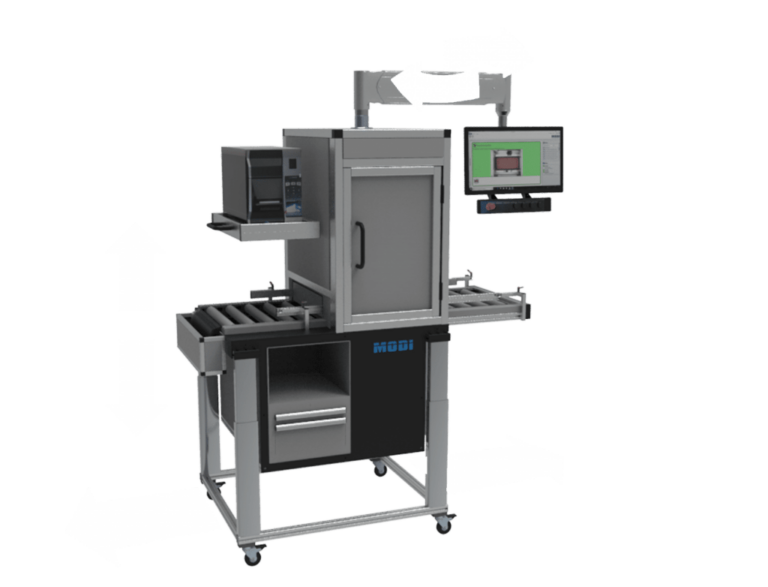
The height of the counting units can be adjusted electronically, so that it is possible to integrate the counting unit into existing production lines without any problems. A simple docking mechanism allows it to be connected to almost any other machine. Trays can therefore be easily moved from system to system.
Lifting and carrying is therefore not necessary.
HSE: The monitor arm can also be rotated and adjusted in height. Each operator can adjust the working position individually. For left-handed users or changing operating situations, the label printer can be turned and the system operated from the other side.
Mobility: Transport rollers allow the counting unit to be moved anywhere in a building.
The most important task of quality control units in the pharmaceutical industry is the testing of active pharmaceutical ingredients, excipients and packaging materials.
The main focal points of GMP (Good Manufacturing Practice) are above all the requirements for hygiene, premises, equipment, documentation and controls. It must therefore be ensured that the necessary checks are carried out in the incoming goods department and that the materials are only released once their quality has been assessed as satisfactory. The GMP rules are laid down in national and international regulations.
The workflows and process steps are digitized in the best possible way to avoid the use of paper in production. You can decide individually what you want to print or just save digitally. This protects the environment and facilitates the workflow!
If all ampoules, syringes or trays had to be counted manually, you can imagine that this would be very time-consuming and error-prone. With the balancing unit, products of all kinds are counted within seconds. The counting results can then be used in customer-specific processes. The use of an automatic balancing unit minimizes error-prone activities.
The table is height-adjustable and can therefore be quickly and easily integrated into any production line. The monitor arm can also be swiveled and is also height-adjustable. This provides an ergonomically optimal working position for every operator. For left-handed users or changing operating situations, the label printer can be turned and the system operated from the other side. (depending on variant)
PROTECT PRODUCTS WITH THE HIGHEST POSSIBLE QUALITY!

Production in accordance with applicable GMP (Good Manufacturing Practice) guidelines is fundamental to MODI in all business areas and therefore part of the corporate culture. With the continuous improvement of our processes with regard to the “EU Guide to Good Manufacturing Practice”, these processes are constantly being transferred to our quality management system.
Our products are manufactured in accordance with GMP rules with the aid of systematic controls, electronic documentation, defined work processes and trained and motivated employees, thus guaranteeing optimum quality.
A GMP-compliant quality management system must fulfill the following requirements:
Qualification of equipment (systems and devices and, if applicable, buildings and premises): In a planned and documented process, it must be demonstrated that the equipment is suitable for the intended purpose and functions reliably under the local conditions (during commissioning and later at regular intervals). This involves going through four phases, each of which requires its own plan and report: Design Qualification (DQ), Installation Qualification (IQ), Functional or Operational Qualification (OQ) and Performance Qualification (PQ).
Validation of processes and methods: In a planned and documented process, it must be shown that the processes (e.g. manufacturing processes) and methods (e.g. analytical methods in quality control) meet the requirements of the product and therefore deliver reliably reproducible results.
Employee training: Employees must receive appropriate training before starting work and the training must be documented.
Risk management and internal audits: The establishment and operation of a functioning risk management system and the performance of regular internal audits (in addition to external audits) are essential for a GMP-compliant quality management system.
First counting unit on the market that can be operated inline with other machines without slowing them down.
No disadvantages due to external factors such as ambient lighting, shadows caused by operators or similar.
No worst-case format recognizable. (Not even with gray caps in gray trays)
Vials and cylinder ampoules can be counted regardless of their size, the size and color of the lid and the size and color of the tray.
8x different cylinder ampoules 2R, 6R, 10R, 10R high, 30R, 5ml, 10ml
8x different vials 1.5ml, 1.7ml, 3ml
5x tray colors
16 cap colors









